 digene HC2 is the Gold Standard
digene HC2 is the Gold Standard
Yesterday. Today. Tomorrow.
Celebrate with us all year long! Don’t miss out on opportunities for exclusive giveaways. Sign up to receive emails and stay in the loop.
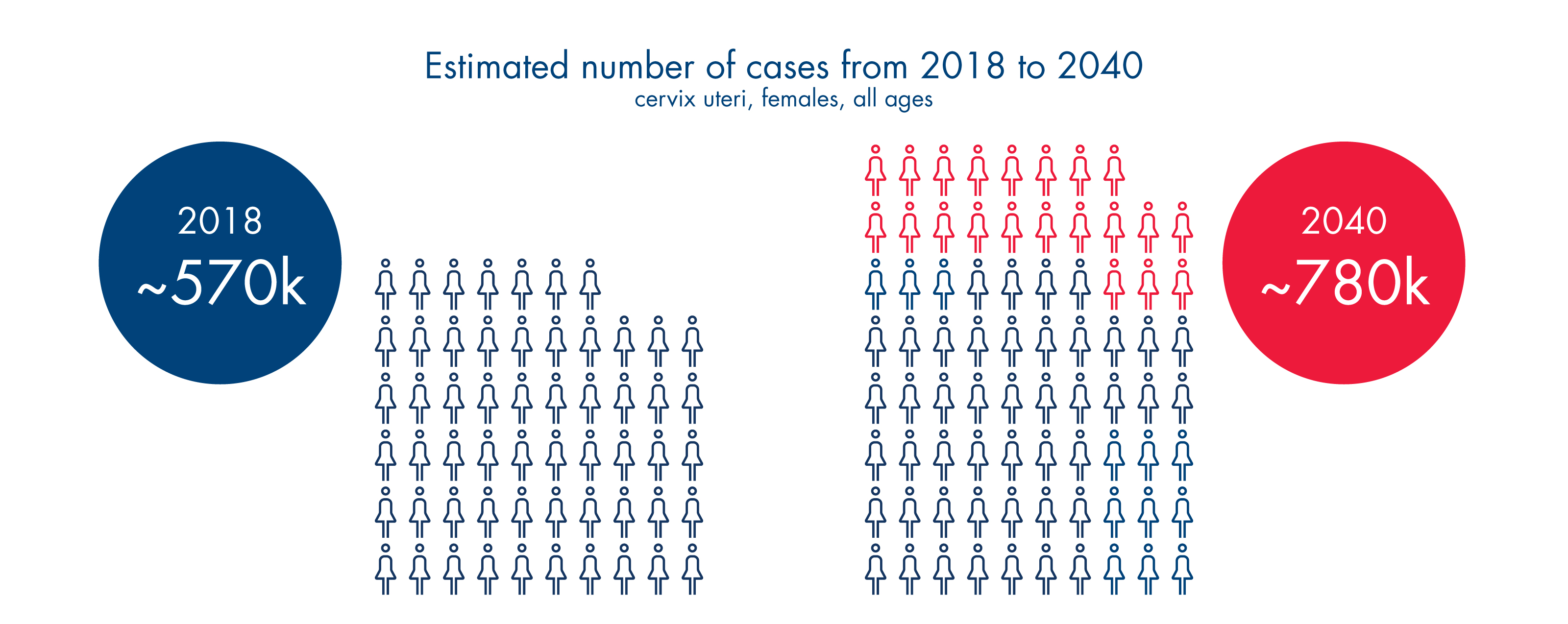
Count me in!Cervical cancer is virtually 100% preventable with vaccination and appropriate screening. As scientists uncover more insights on cervical cancer and with the development of new tests and guidelines, we are entering into an age with the real possibility of eliminating one of the highest burden cancer affecting women globally.
QIAGEN has been committed to this cause for over two decades. The digene HC2 High-Risk HPV Test is the gold standard for HPV testing worldwide and QIAGEN continues to bring new innovations to the market with the QIAsure Methylation Test and the QIAscreen HPV PCR Test.
Let’s brush up on the latest cervical cancer facts and figures. And more importantly, let’s share this information with those close to us who may be at risk.
There is a pressing need to deliver effective screening tests to these countries to successfully prevent cervical cancer.
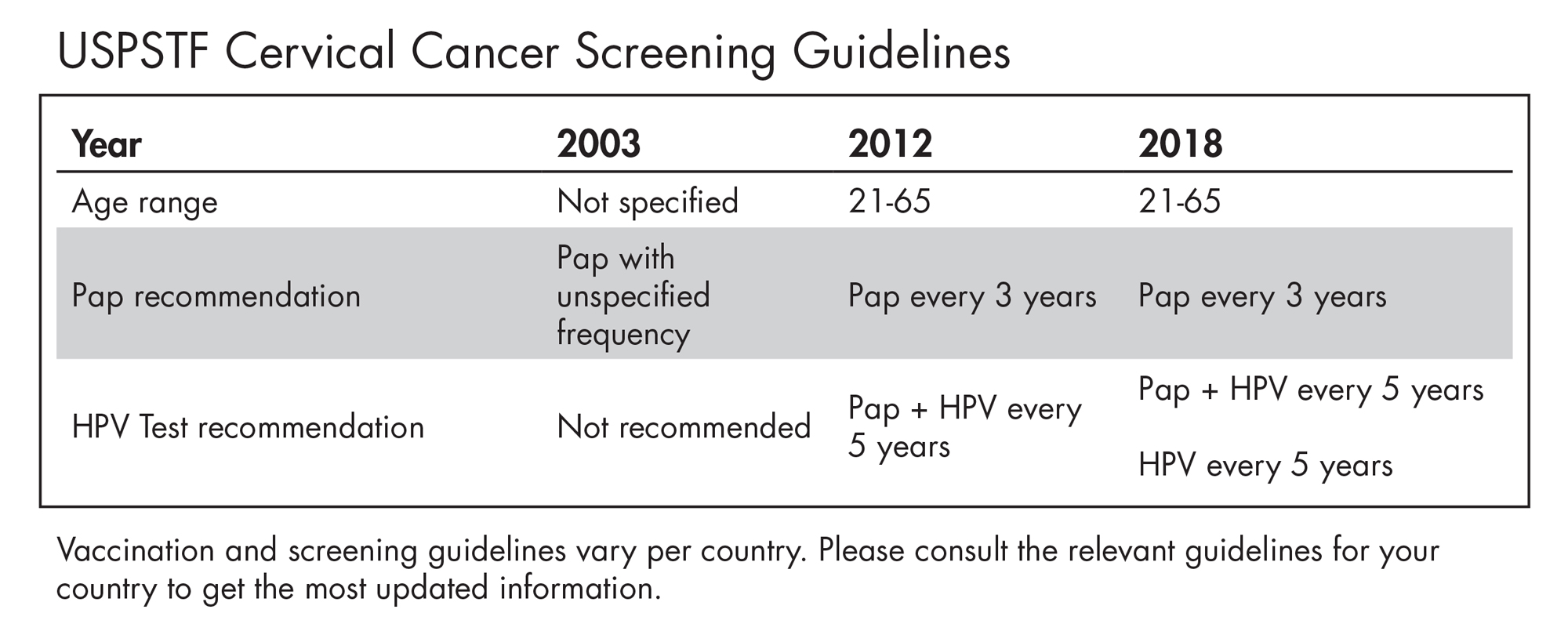
Cervical cancer is one of the few cancers that can be prevented with vaccination and early detection. As HPV testing becomes widely accepted, cervical cancer screening guidelines are changing to reflect the shift in clinical practice. QIAGEN is proud that the digene HC2 test generated much of the data to support the guidelines over the last 20 years.
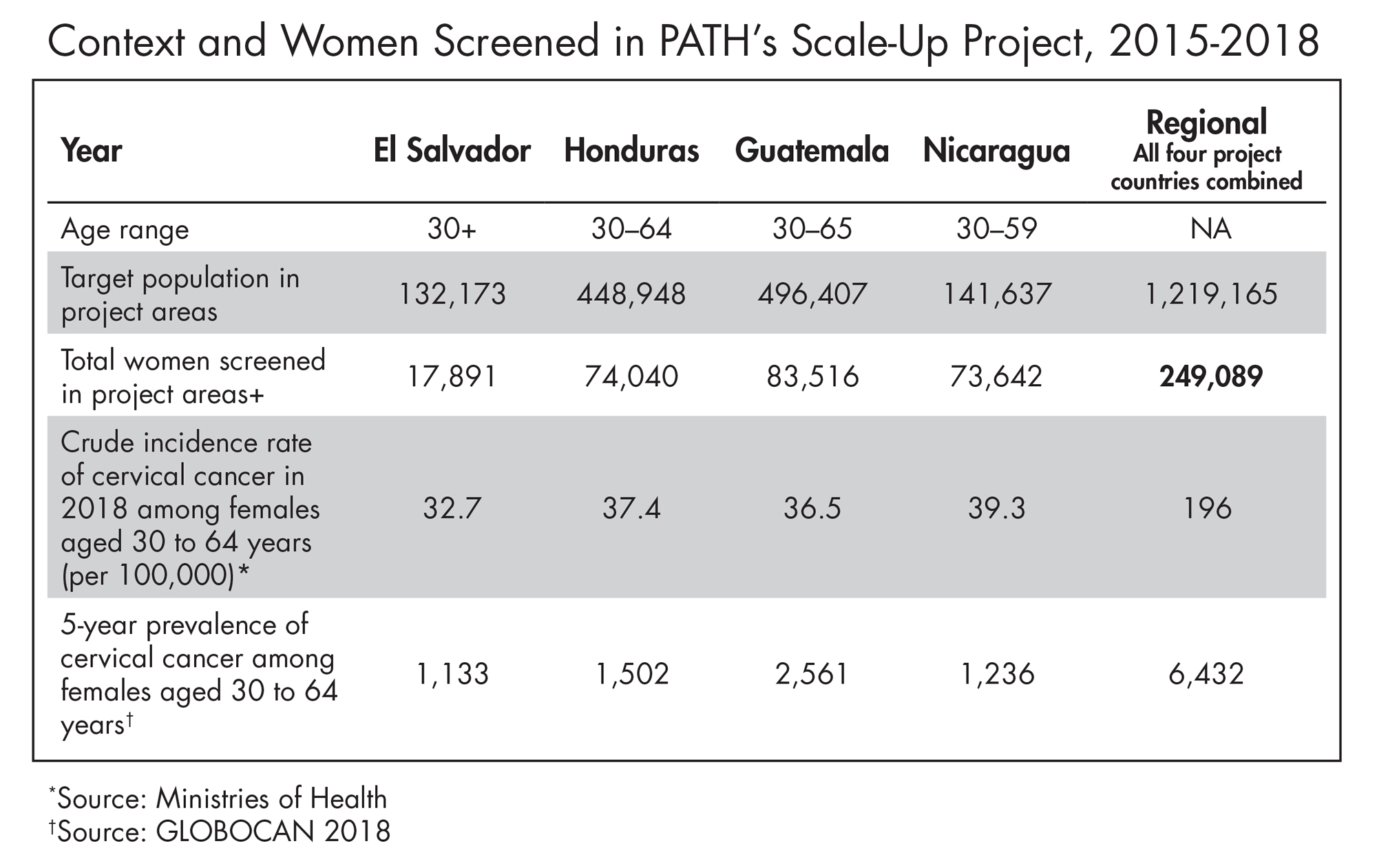
From 2014–2018, through the Scale-Up project, PATH and local partners provided technical assistance to governments in Nicaragua, Guatemala, Honduras and El Salvador to introduce HPV testing and improve access to screening. PATH encouraged ministry of health leadership to implement self-sampling as a primary screening strategy to enhance access and acceptability among women while overcoming infrastructure barriers. To date, almost a quarter of a million women have been screened using QIAGEN’s careHPV® Test across the four countries.
 digene HC2 is the Gold Standard
digene HC2 is the Gold StandardCelebrate with us all year long! Don’t miss out on opportunities for exclusive giveaways. Sign up to receive emails and stay in the loop.
Count me in!1. Schiffman, M. et al. (2007) Human papillomavirus and cervical cancer. The Lancet 370, 890–907.
2. U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999-2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, June 2018.
3. World Health Organization Global Cancer Observatory – Cancer Tomorrow. GLOBOCAN 2018. http://gco.iarc.fr/tomorrow/graphic-isotype