It’s an exciting time for cervical cancer screening.
As scientists uncover more insights on cervical cancer, new tests and guidelines have emerged in clinical practice for cervical cancer prevention and screening.
January is Cervical Health Awareness month in the US and other parts of the world. Let’s brush up on the latest cervical cancer knowledge and share with those close to us who may be at risk.
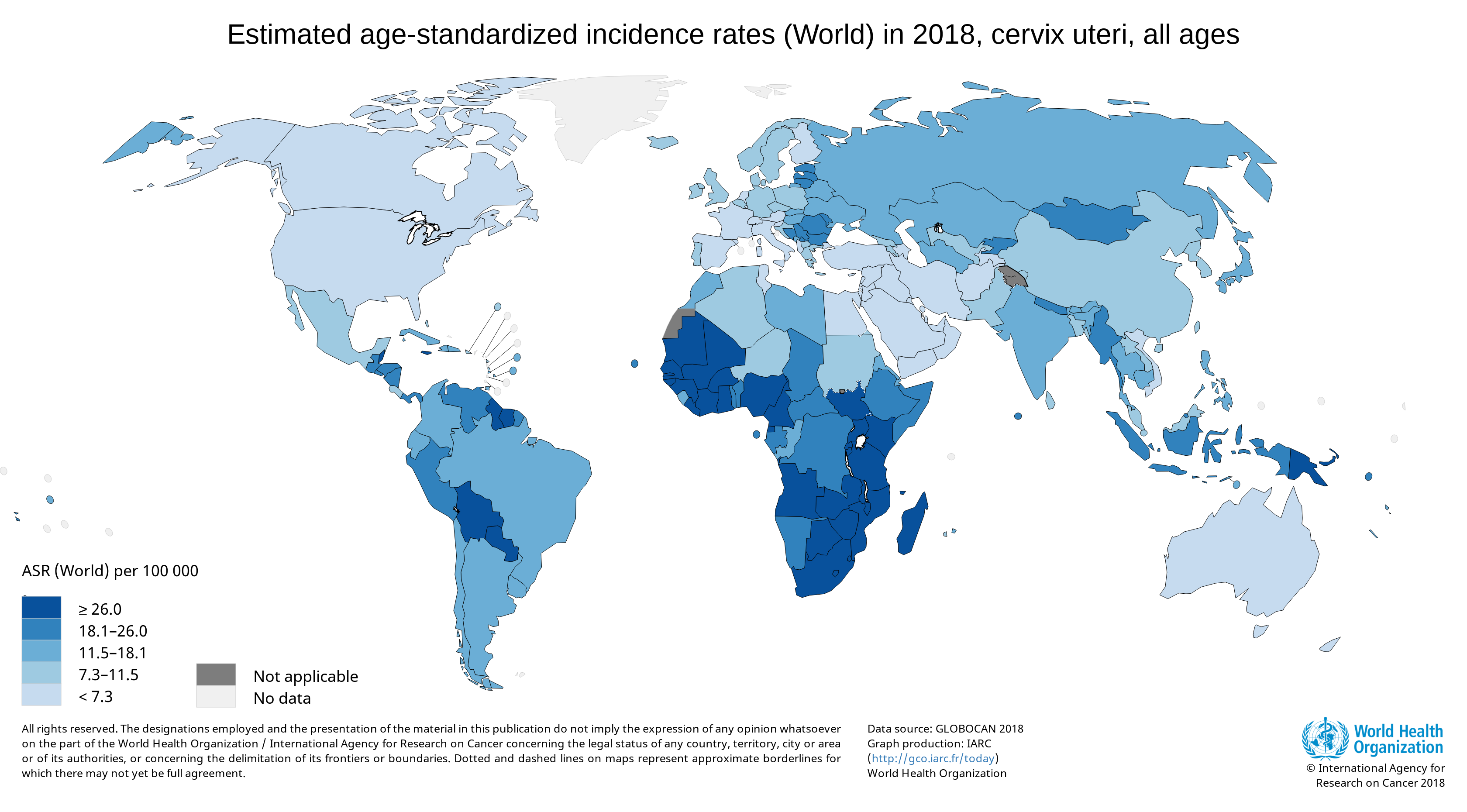
The global burden of cervical cancer
Cervical cancer is the 2nd most frequent cancer worldwide, with 80% of cases in low and middle income countries (LMICs) where adequate screening options are not available. (1) In the United States, more than 12,000 women will be diagnosed with cervical cancer each year, and more than 4,000 women will die from cervical cancer. (2)
The development of cervical cancer screening
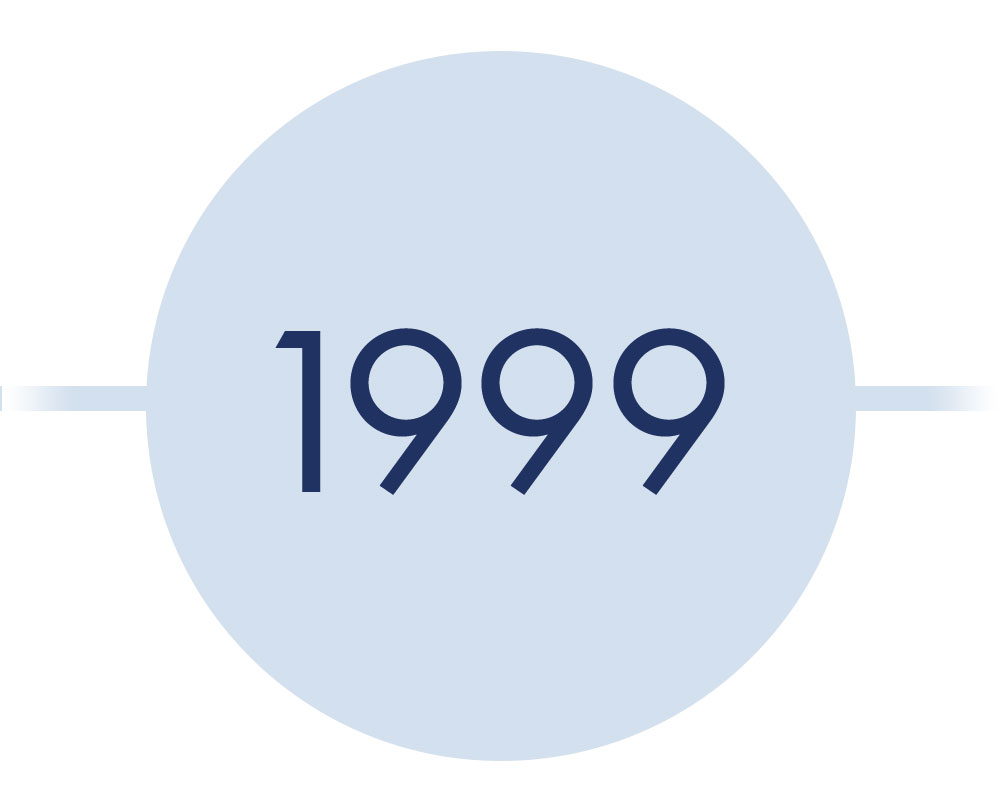
*year should be 1949*
The Papanicolaou (Pap) Test
- Introduced by George Papanicolaou
- A significant step that reduced cervical cancer rates
- However, it could not detect the agent of cervical cancer
*should say early 1980s*
HPV caused cervical cancer
- Discovered by Harald zur Hausen
- HPV-16 and HPV-18 DNA was found in cervical tumor samples
- Zur Hausen won the Nobel Prize in Medicine in 2008 for this discovery

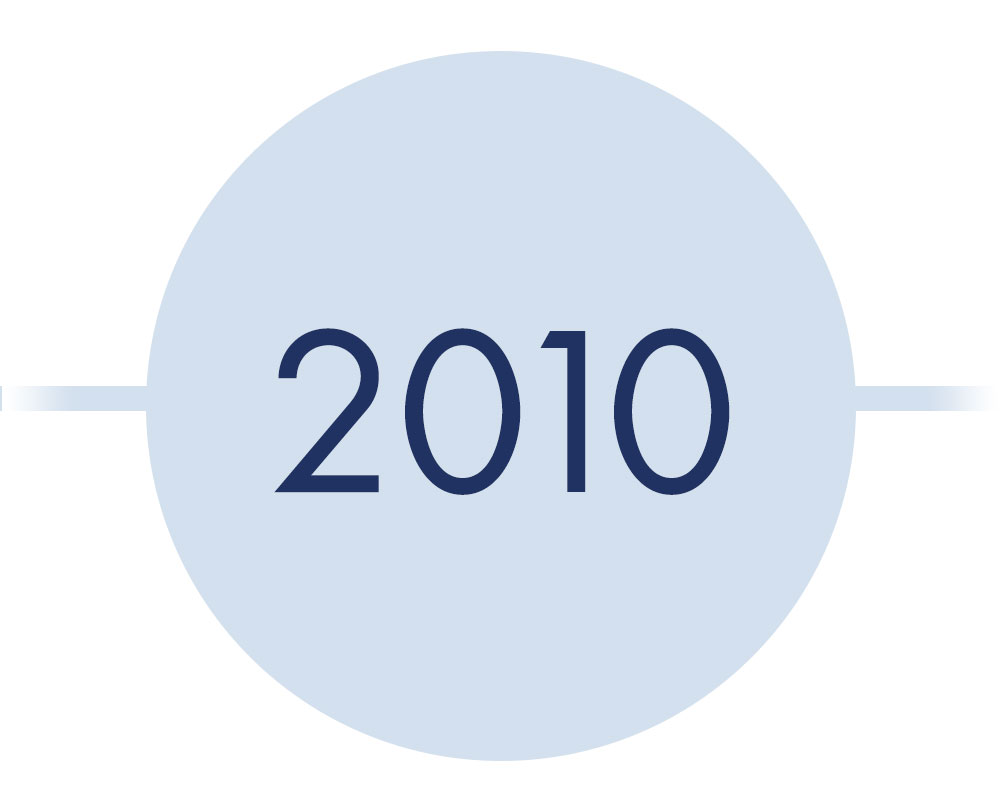
*year should say 1988-1999*
digene HC2 HPV Test
- Created and developed to detect HPV DNA
- Utilized RNA probes complementary to the full HPV genome
- Optimized sample collection and manufacturing

*year should say 1999 and 2003*
FDA approval for digene Test
- First HPV DNA test approved by the FDA
- A new type of cervical cancer screening test for women
- Approved for ASCUS reflex testing and cotest with Pap
The digene HC2 High-Risk HPV Test has become the gold standard for HPV testing worldwide. Now, we know that ~99% of cervical cancer cases are caused by the human papillomavirus (HPV), which has over 100 genotypes. The HPV-16 and HPV-18 genotypes, often referred to as high-risk HPV types, are responsible for over 70% of cervical cancer. HPV DNA tests target hrHPV genotypes to identify women at risk for developing cervical cancer.
The future of cervical cancer screening with QIAscreen
QIAscreen provides a menu of applications and flexibility in sample input options for HPV testing. Watch the video to find out more about the new QIAscreen test.
Introducing QIAscreen, a new HPV PCR screening solution.
The QIAscreen HPV PCR Test is an in vitro real-time PCR-based assay for the qualitative detection of human papillomavirus (HPV) DNA of 15 (likely) (1) high-risk HPV genotypes.
QIAscreen can bolster your Rotor-Gene Q workflow, providing a menu of applications on one instrument.
QIAscreen rounds out QIAGEN’s family of PCR-based women’s health tests on the Rotor-Gene Q instrument, which include tests for C. trachomatis, N. gonorrhoeae, and T. vaginalis. Labs can conduct a panel of women’s health tests in a more streamlined and resourceful manner to provide comprehensive insights for clinicians and patients.
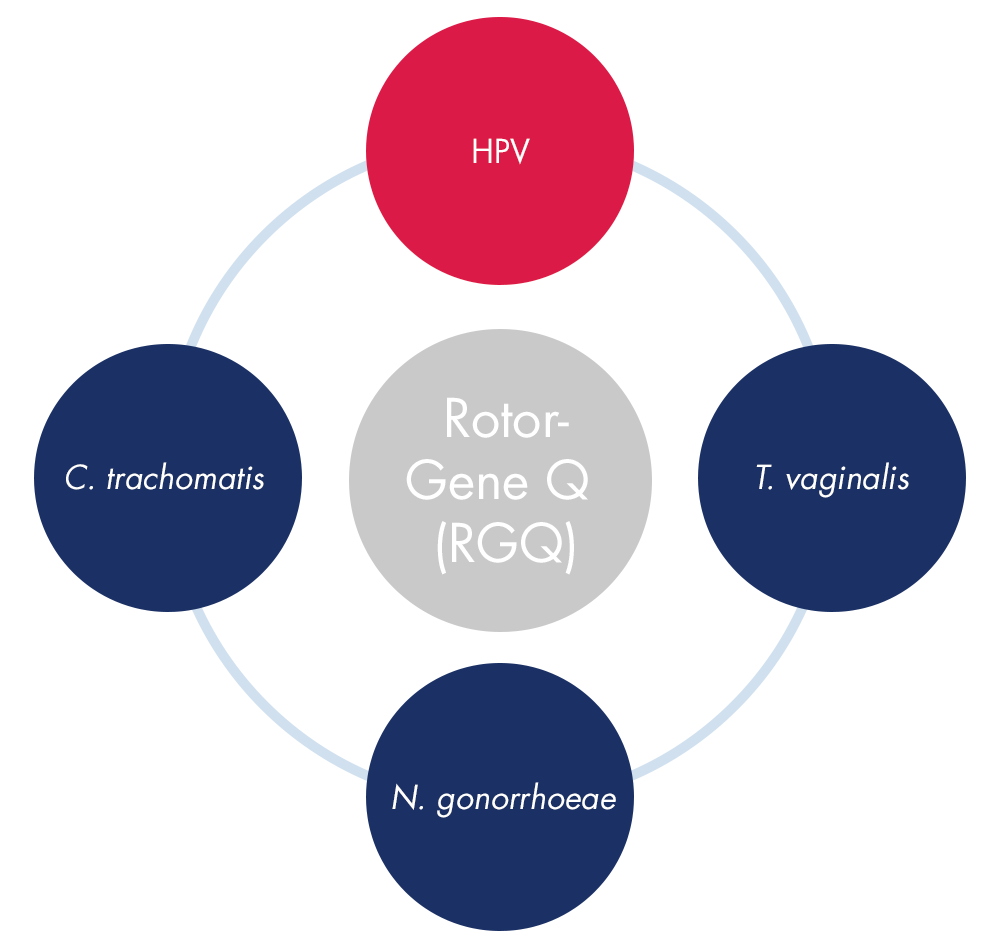
In addition, QIAscreen has several unique features:
-
Total HR-HPV Detection
QIAscreen detects 15 HPV types: 14 identified high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) and 1 likely high-risk HPV type (67). -
Unique HPV targeting
QIAscreen is the only HPV PCR test targeting the E7 gene. The viral E6/E7 region is invariably retained in integrated viral genomes in cervical cancers (2). -
Compatible with self sampling
QIAscreen was validated for many sample types, including physician-taken samplings and self sampling (i.e. lavage and brush) -
Flexible workflow
QIAscreen’s open-ended sample extraction works with all common methods, such as the QIAsymphony system.
References
1) International Agency for Research on Cancer. (2009). A review of human carcinogens. Part B: Biological Agents. Monographs 100B.
2) Snijders, P.J., Steenbergen, R.D., Heideman, D.A., Meijer, C.J. (2006) HPV-mediated cervical carcinogenesis: concepts and clinical implications. J. Pathol. 208, 152.
3) QIAscreen HPV PCR Test Instructions for Use. QIAGEN, 2018.
The QIAscreen HPV PCR Test is intended for in vitro diagnostic use in Europe. Self-screen B.V. is the legal manufacturer of the QIAscreen HPV PCR Test.
QIAGEN HPV solutions are used globally to fight cervical cancer
From 2014–2018, through the Scale-Up project, PATH and local partners provided technical assistance to governments in Nicaragua, Guatemala, Honduras and El Salvador to introduce HPV testing and improve access to screening. PATH encouraged ministry of health leadership to implement self-sampling as a primary screening strategy to enhance access and acceptability among women while overcoming infrastructure barriers. To date, almost a quarter of a million women have been screened using QIAGEN’s careHPVTM Test across the four countries.
Context and Women Screened in PATH’s Scale-Up Project, 2015-2018
| El Salvador | Honduras | Guatemala | Nicaragua | Regional (all four project countries combined) | |
| Age range | 30+ | 30–64 | 30–65 | 30–59 | |
| Target population in project areas | 132,173 | 448,948 | 496,407 | 141,637 | 1,219,165 |
| Total women screened in project areas+ | 17,891 | 74,040 | 83,516 | 73,642 | 249,089 |
| Crude incidence rate of cervical cancer in 2018 among females aged 30 to 64 years (per 100,000)* | 32.7 | 37.4 | 36.5 | 39.3 | 196 |
| 5-year prevalence of cervical cancer among females aged 30 to 64 years* | 1,133 | 1,502 | 2,561 | 1,236 | 6,432 |
+Source: Ministries of Health
*Source: GLOBOCAN 2018
- India, Barshi Project: This project is a multicentre follow up study established in 2016 where researchers are following up on a HPV-vaccinated population. Around 2000 women have been reflex tested using the digene HPV Genotyping PS test.
- India, Adyar Cancer Institute: In 2014, this was the first site in India to implement a community-based screening project to screen rural women in Villupuram (Tamil Nadu) using the careHPV test. This project expands screening to two more districts in the state. They have screened around 25,000 women and aim to have careHPV as the primary method for screening.
- Myanmar, Central Women Hospital: This project aims to have routine screening using the careHPV test. To date, they have screened more than 1000 women.
- Sri Lanka, Rottary Club: This project is a collaboration with a local hospital to implement routine screening using the careHPV test. To date, they have screened 2000-3000 women.
- South Africa, University of Cape Town: This project uses the digene HC2 Test to analyze self-collected and physician-collected samples of 1200 women.
- University of Pretoria: This project uses the digene HC2 Test to analyze nurse-collected samples of 2000 women.
